Quality infrastructure is an essential foundation for success in radiopharmaceutical and cell & gene therapy manufacturing. To meet modern industry expectations, companies must transition away from paper-based or outdated systems and adopt electronic Quality Management Systems (eQMS). This shift is critical for scalability, compliance, and data integrity.
When data is misplaced, inaccessible, or non-compliant, the drug development process faces severe delays and increased risks. If data integrity is compromised, clinical trials may fail, and regulatory approval becomes unattainable. Non-compliance in any aspect—people, processes, or products—can prevent regulatory approval and hinder life-saving therapies from reaching patients.
Both radiopharma and other novel therapies—like cell & gene therapies (CGT)—present unique challenges in quality infrastructure, requiring specialized approaches to quality management.
Understanding the Nuances: Radiopharma vs. Cell & Gene Therapy
Compliance gaps in cell and gene therapies and Radiopharmaceuticals are commonly due to the complexity of regulations, evolving standards, and the highly specialized nature of these products. They may present as errors in regulatory documentation or data integrity, gaps in chain of custody or chain of identity tracking (especially for autologous therapies), or non-compliance with emerging regulations.
Radiopharmaceuticals
Developing radiopharmaceuticals requires careful consideration of both drug development and the handling of radioactive isotopes. These therapies involve specialized production methods, strict handling procedures, and rigorous quality control measures to ensure efficacy and patient safety. Key steps—including synthesis, labeling, testing, and administration—must adhere to stringent regulatory guidelines.
The short half-life of many isotopes demands rapid production, quality control, and release. To meet these challenges, companies need GMP-compliant operations, a robust quality system, and an effective eQMS. Implementing these elements ensures regulatory compliance, minimizes errors, and maintains data integrity, leading to the efficient and timely delivery of radiopharmaceuticals for patient use.
Cell & Gene Therapies
Manufacturing cell and gene therapies presents unique patient-specific challenges, including stringent chain-of-identity and chain-of-custody tracking. Like Radiopharmaceuticals, adhering to Good Manufacturing Practices (GMP), establishing a robust quality system, and implementing a comprehensive eQMS are essential for managing these complexities.
GMP guidelines ensure the safety and quality of manufactured products by covering all aspects of production, including facilities, equipment, and personnel. Compliance with these standards is required for all pharmaceutical manufacturers in the United States. For advanced therapy companies, implementing an eQMS is a crucial first step in ensuring regulatory alignment and quality assurance.
Is It Time to Implement an eQMS?
Transitioning to an electronic Quality Management System (eQMS) is a critical step for life sciences and manufacturing companies seeking to modernize operations and maintain regulatory compliance. However, the road to successful implementation is often met with significant barriers. One of the most common challenges is resistance to change—especially from teams accustomed to paper-based processes or legacy systems. This cultural hurdle can slow down adoption and reduce the overall effectiveness of the transition if not addressed early with strong change management and leadership support.
Another key obstacle is the cost of implementation. Adopting an eQMS typically involves a substantial upfront investment in software licenses, IT infrastructure, and employee training. Even after go-live, companies must account for ongoing expenses such as system updates, support contracts, and potential customization work to tailor the platform to their specific processes. These financial considerations can be especially daunting for smaller organizations or those with limited resources.
Regulatory compliance and system validation present additional complexity. Companies must ensure that their eQMS meets strict industry standards such as FDA 21 CFR Part 11, ISO 9001, and GMP. Validating the system to demonstrate it meets these requirements is not only time-consuming but also demands deep expertise to ensure accuracy and audit readiness. This becomes even more complicated when organizations try to integrate the eQMS with existing systems, including ERP, CRM, PLM, or older QMS tools. Seamless integration is crucial for maintaining data continuity, but it often comes with technical hurdles—particularly in data migration, where ensuring the accuracy and completeness of historical quality records is critical.
Training employees to use the new system effectively is another area where companies can stumble. Without adequate onboarding and role-specific training, users may struggle to engage with the eQMS, limiting its impact. Additionally, customization and scalability can become pain points if the selected solution doesn’t align with existing workflows or can’t adapt to future growth. Some platforms require extensive configuration to match business needs, and not all are built to scale with a growing operation.
Security is also a growing concern, as companies must protect sensitive quality and compliance data from breaches. Robust access controls, encryption, and audit trails are essential for maintaining data integrity and trust. Finally, the success of an eQMS project often hinges on vendor selection and reliability. Choosing a partner who not only understands the regulatory environment but also offers long-term support is vital to avoid vendor lock-in and future disruptions.
A Phased Approach to Implementing eQMS in Advanced Therapies
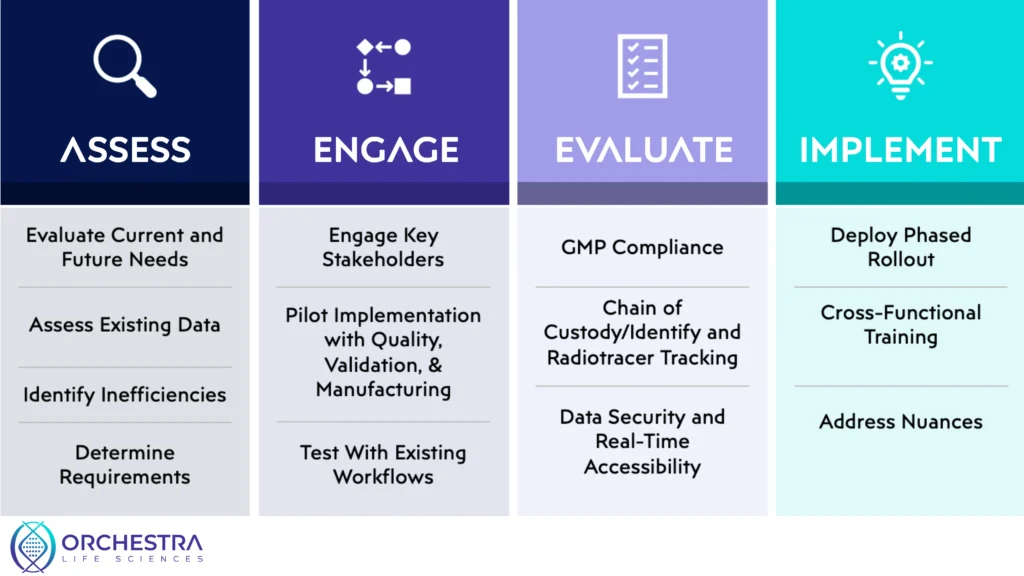
Step 1: Assess Current and Future Needs
The first step in transitioning to an electronic Quality Management System (eQMS) is a comprehensive assessment of your organization’s existing data management infrastructure. This involves identifying gaps, redundancies, or inefficiencies in how quality and compliance data are currently captured, stored, and accessed. Beyond addressing today’s pain points, companies must also consider long-term needs—particularly in areas like real-time release testing, automated batch record management, and readiness for increasingly complex regulatory demands. For organizations in fast-moving, high-compliance fields such as cell and gene therapy (CGT) or radiopharmaceuticals, building a system that supports scale, agility, and precision is essential from the outset.
Step 2: Engage Key Stakeholders
Successful eQMS implementation depends on strong alignment across departments. Early and ongoing engagement with key stakeholders—particularly from executive leadership, regulatory affairs, and quality control—is critical to securing the buy-in and budget needed for adoption. Launching a pilot phase with high-impact groups, such as validation teams, QC labs, or manufacturing operations, allows organizations to demonstrate the system’s value and work out any early-stage issues. Ensuring that the new system integrates smoothly with clinical workflows and regulatory documentation processes can also help minimize resistance and maintain compliance during the transition.
Step 3: Vendor Evaluations
Choosing the right eQMS vendor goes far beyond software features—it requires selecting a long-term partner that understands the nuances of regulated manufacturing. For companies producing small-batch, high-value therapies, especially in CGT or nuclear medicine, GMP compliance is non-negotiable. Vendors must also support full end-to-end traceability, including chain of custody and chain of identity tracking in CGT, as well as radiotracer tracking in radiopharmaceutical workflows. Additional must-haves include robust data security protocols, real-time accessibility across distributed manufacturing sites, and flexible reporting capabilities to support both internal oversight and external audits.
Step 4: Implementation Strategy
A thoughtful, phased implementation strategy helps reduce operational disruptions and allows time for teams to adapt. Rather than attempting a full system switchover all at once, organizations should consider rolling out the eQMS in stages—starting with lower-risk modules and gradually expanding to critical functions. Cross-functional training is essential to ensure that manufacturing, quality assurance, and regulatory teams can fully leverage the new system. Industry-specific challenges must also be addressed early: this includes managing the ultra-short shelf life of radiopharmaceutical products, enabling closed-loop manufacturing for CGT, and meeting radiation safety and handling requirements in nuclear medicine environments. A flexible and well-supported implementation plan ensures both compliance and continuity.
Partnering with Experts for a Seamless Transition
To ensure a smooth transition to an eQMS, companies should leverage expertise in regulatory compliance, automation, and advanced therapy manufacturing. By aligning with industry best practices and ensuring future scalability, organizations can optimize their quality infrastructure for long-term success.
Investing in quality infrastructure is essential for regulatory success, operational efficiency, and patient safety. Companies that proactively adopt eQMS not only meet regulatory expectations but also improve data integrity, operational efficiency, and scalability ultimately accelerating patient access to life-saving therapies.

